First, the EU’s legislative priorities for the next two years are summarised (see A.). The Commission Recommendation “Safe and sustainable by design” is then presented (see B.), before the individual legal acts REACH (see C.), CLP (see D.), Biocidal Products Regulation (see E.) and POPs (see F.) are examined in detail.
A. EU legislative priorities for 2023 and 2024
Under 23.12.2022, the European Parliament, the Council and the European Commission presented a joint declaration on the EU’s legislative priorities for the next two years (2022/C 491/01). Among other issues, the implementation of the “Green Deal” and related legislative projects remains a top political priority. Although the individual issues subsumed under this objective remain diverse, they include in particular aspects of chemicals regulation such as microplastic pollution or sustainable product design. The fight against environmental crime is also to be tackled as a top priority. In this context, the revision of the Environmental Criminal Law Directive 2008/99/EC also aims, among other things, at introducing or tightening sensitive sanctions for violations of REACH, BPR, RoHS and other sets of regulations.
B. Commission Recommendation “Safe and sustainable by design
As early as 08.12.2022, the Commission presented Recommendation (EU) 2022/2510 on the establishment of a European framework for “inherently safe and sustainable” (SSbD) chemicals and materials. Primarily, this is only a framework to set the course for research and innovation projects and thus create the basis for incentives by the EU and the Member States in research, science and industry. Nevertheless, the framework will have a direct impact on aspects of chemicals regulation insofar as the criteria for safety and sustainability assessments are already defined. The starting point for any such assessment is the determination of the hazard properties of the substances or materials on the basis of the requirements of CLP. It is noteworthy that the Commission also explicitly allows the use of criteria for hazard properties that are not (yet) introduced as hazard classes under CLP, such as PBT, vPvB, PMT, vPvM or ED. Irrespective of this, all hazard classes according to CLP are taken into account. However, not every hazard property necessarily means that a substance to be classified accordingly would not be inherently safe or sustainable. This is because the assessment also requires the examination of health and safety aspects during manufacture and distribution (Step 2), health and environmental aspects during final use (Step 3) and environmental sustainability (Step 4).
Even though the Commission Recommendation emphasises that the safety and sustainability assessment in particular has no impact on the legal obligations in the Union applicable to chemicals and materials, consideration of the findings will also and especially be expected in the definition and further development of the legal framework. In particular, it remains to be seen whether and in what way the findings from corresponding assessments will be taken into account, for example, within the framework of the design of exemptions from authorisation obligations or restrictions pursuant to REACH. But also the evaluation of exemption applications according to RoHS, the design of procedures according to BPR or also the definition of specifications according to the legal framework for eco-design requirements, which is currently being revised, can be expected.
C. REACH
I. Revision
As already reported at the beginning of last year, one of the most far-reaching changes is pending with the revision of the REACH Regulation. The main objectives of an amendment of the REACH Regulation follow the “Chemicals Strategy for Sustainability”. Consequently, the focus will be on measures for the targeted implementation of requirements for so-called “safe and sustainable by design chemicals”, the introduction of “non-toxic material cycles” and the establishment of an “essential use concept”. In addition, the most hazardous substances as well as endocrine disruptors will be considered, as well as evaluation criteria for mixtures.
Studies commissioned in connection with the revision have largely been completed, whereby the evaluation of a reform of authorisations and restrictions as well as the support regarding the impact assessment are still open. After various delays, the draft of the intended amendments is expected in the course of this year. The adoption of a corresponding proposal by the Commission is now expected for the last quarter of 2023 according to the Commission Work Programme for 2023 of 18.10.2022.
II. Substance and dossier evaluation
According to Art. 41 para. 5 REACH, ECHA has to select a percentage of dossiers corresponding to at least 20% of the total number of dossiers submitted to the Agency for registration in tonnage bands of 100 tonnes or more per year for the evaluation of compliance with the requirements for registration dossiers by 31 December 2023. The “REACH Evaluation Joint Action Plan” already follows this approach.
ECHA has submitted the draft “Community Rolling Action Plan (CoRAP)” update for the years 2023-2025 under 13.12.2022. This contains 24 substances, five of which are to be evaluated in 2023 and a further 19 in 2024 and 2025.
III. Candidate list for authorisation
As expected, ECHA updated the candidate list under 17.01.2023 and included nine additional substances:
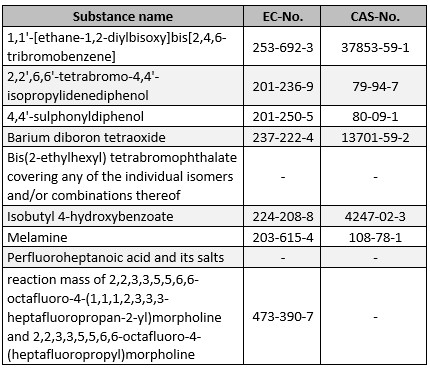
The list now comprises 233 entries, which must be taken into account directly both for communication in the supply chain according to Art. 33 REACH and for notifications in the SCIP database. For any notification obligations according to Art. 7 para. 2 REACH, a transitional period of another six months will initially apply to the substances newly included as of 17.01.2023 (cf. Art. 7 para. 7 REACH).
In line with ECHA’s usual practice, a further addition to the candidate list is expected in the middle of the year.
IV. Applications for authorisation
For a number of substances subject to authorisation under Annex XIV to REACH, the deadline for submission of authorisation applications ends in the current year. Affected are:
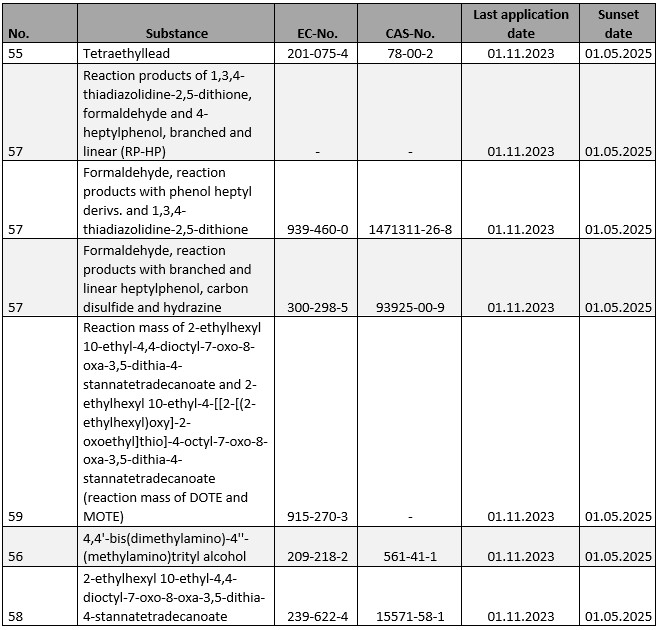
Without a timely application, the substances in question may only be used until the respective sunset dates. This also applies if an application for authorisation is submitted after the last application deadline. In this case, the authorisation decision must first be awaited before the corresponding use may be resumed. The same applies to those substances for which the corresponding sunset dates have been recorded in the current year without corresponding applications for authorisation having been submitted in time (cf. for details the list of substances subject to authorisation).
For DEHP, BBP, DBP and DIBP, specific transitional provisions for authorisation applications concerning specific uses according to Nos. 4 to 7 in Annex XIV REACH will end in 2023.
In addition, the supplementary transitional period for authorisation-relevant expiry dates ends on 01.03.2023. Exceptions exist here for the use of substances in the manufacture of spare parts as articles or as complex objects for the repair of articles or complex objects whose manufacture has ceased or will cease before the expiry date specified in the entry for the respective substance, if the substance was used in the manufacture of these articles or complex objects and they cannot function properly without the spare part and the spare part cannot be manufactured without this substance. The complementary transitional period also applied to the use of the substance (as such or in a mixture) for the repair of such articles or complex objects if the substance as such or in a mixture was used in the manufacture of these articles or complex objects and they can only be repaired using this substance (cf. Note 2 to Annex XIV to REACH inserted under Regulation (EU) 2020/171).
V. Restrictions
For a number of restrictions, existing exemptions are expiring:
- For example, the use of lead in shot ammunition will be subject to more stringent requirements from 15.02.2023 (cf. No. 63 in Annex XVII to REACH).
- For linear and branched perfluorinated carboxylic acids and related substances according to No. 68 in Annex XVII to REACH, bans on manufacture, placing on the market and use will apply from 25 February 2023. In addition, various transitional arrangements expire in 2023 for this group of substances.
- Under entry no. 72 in Annex XVII to REACH, a limit of 300 mg/kg will no longer apply to the placing on the market of formaldehyde (CAS 50-00-0) in jackets, coats or upholstery from 01.11.2023, but the concentration of 75 mg/kg specified in Annex 12.
- For diisocyanates (O = C=N-R-N = C=O, where R is an aliphatic or aromatic hydrocarbon unit of any length), far-reaching bans on industrial and professional use will take effect from 24.08.2023, including the obligation for employers to ensure sufficient training on the safer use of diisocyanates (cf. entry no. 74 in Annex XVII to REACH).
- Already since 04.01.2023 the transitional period for the use of Pigment Blue 15:3 (CI 74160, EC 205-685-1, CAS 147-14-8) and Pigment Green 7 (CI 74260, EC 215-524-7, CAS 1328-53-6) in mixtures for use in tattooing has expired.
- According to entry No. 76 in Annex XVII to REACH, N,N-dimethylformamide (CAS 68-12-2, EC 200-679-5) is subject to a ban on manufacture and use at concentrations ≥ 0.3 % from 12.12.2023.
Furthermore, the proposal on the restriction of PFAS submitted by Germany, Denmark, the Netherlands, Norway and Sweden on 13.01.2023 deserves special attention. It is expected that the proposal will aim at restricting the production, placing on the market and use of PFAS. The substance group of PFAS according to the OECD definition comprises more than 4,500 substances, so that the restriction is likely to affect a large number of uses, products and industries. This has already been confirmed in the previous consultations. The Member States responsible for the restriction proposal assume that the group of substances contributes to global contamination of surface water, groundwater, drinking water and soil, in particular due to their persistence, water solubility and mobility. Removal measures would have proved very difficult and extremely costly.
The proposal will be published by ECHA on 07.02.2023. The Committee for Risk Assessment (RAC) and the Committee for Socio-economic Analysis (SEAC) will deliberate as early as March 2023 on whether the submitted restriction proposal meets the legal requirements under REACH. After that, the committees are to begin the scientific examination of the proposal. According to current plans, a six-month public consultation is also to start from 22.03.2023. It remains to be seen whether and to what extent the submitters of the dossier propose exemptions from the proposed restrictions. Affected industries and companies are, however, already well advised to prepare for the later consultation process and to develop suitable, risk-related or socio-economic justifications in order to work towards the adoption of appropriate exemptions. Further details can be found in ECHA’s press release.
Various consultation processes are also still underway for further restrictions, on which submissions can still be made in 2023.
VI. Further risk management measures
Once again, a focus in the implementation of the REACH Regulation in the current year will be on the continuation of ECHA’s integrated regulatory strategy. The regulatory needs assessment list published by ECHA currently contains 2,236 individual entries (as of 22.12.2022). It is therefore to be expected also for 2023 that ECHA will contact affected registrants of substances for which new risk management measures are expected. On a regular basis, registrants will be asked to update their registration dossiers and review the use information. In addition, registrants will be enabled to anticipate the possible impact of the proposed measures.
VII. Ongoing consultation process
In line with the wide range of potential regulatory approaches, companies and associations should continue to follow the various consultation processes with great care and attention in 2023. This applies in particular to calls for comments and evidence, future restrictions and authorisation requirements, as well as any experimental proposals. Only with targeted participation in the corresponding consultation procedures on the part of the industry regulatory undesirable developments can potentially be avoided.
VIII. Enforcement measures
As part of the eleventh major enforcement project of the Forum for Exchange of Information on Enforcement, a network of authorities responsible for the enforcement of the REACH, CLP and PIC regulations in the EU as well as in Norway, Iceland and Liechtenstein, the focus in 2023 will be on the review of safety data sheets, also with a view to the new requirements under Annex II to REACH.
D. CLP
In the current year, the proposed revision of the CLP Regulation together with the supplementary introduction of new hazard classes (EDs, PBTs, PMTs, etc.) by delegated act deserves special attention. Especially the Commission’s proposal for the introduction of new hazard classes has to be critically assessed against the background of the limited legislative competences of the Commission according to Art. 53, Art. 53a CLP. The Commission is only empowered under the CLP Regulation to adopt delegated acts under Art. 53a CLP to amend Art. 6 para. 5, Art. 11 para. 3, Art. 12 and 14, Art. 18 para. 3 (b), Art. 23, Art. 25 to 29, Art. 35 para. 2 subpara. 2 and 3 and Annexes I to VIII for adaptation to technical and scientific progress, taking due account of the further development of the GHS, in particular any UN amendments concerning the use of information on similar mixtures, and taking into account developments in internationally recognised chemical programmes and accident database data. The amendment of the CLP Regulation to introduce new hazard classes does not fall under these powers.
In addition, the European Court of Justice (ECJ), in its judgment of 23.11.2022, declared the classification of titanium dioxide by the EU Commission to be null and void under Article 263 TFEU (we have reported here). The interpretation of the concept of intrinsic properties of substances, on which the classification according to CLP is based, is likely to be significant, especially in view of the introduction of the new hazard classes for “mobile” substances (PMT, vPvM).
ECHA has also launched a series of public consultation proposals for harmonised classification and labelling, which will expire in 2023.
E. Biocides
For the current year, the focus is on the continuation of procedures concerning the approval of active substances. However, the originally announced completion of the work programme by 2024 will probably not be met. It is therefore to be expected that questions of how to cope with the workload from the current work programme and its continuation after 2024 will move into the centre of attention during the current year.
I. Product approvals
Within the scope of Regulation (EU) No 528/2012 (BPR), the active substance approval period for the active substance L-(+)-lactic acid for product type (PT) 6 will start on 01.11.2023. By this date, the companies concerned must submit corresponding applications for authorisation of biocidal products in order to be able to make use of further transitional periods (cf. Article 89 of the CPD).
II. Notification of biocidal products
Since 01.01.2022, the notification procedure according to the Ordinance on the Implementation of Biocidal Products Law (ChemBiozidDV, BGBl. I 2021, No. 57) applies to biocidal products. By 31.03.2023, manufacturers or importers of a biocidal product made available on the market in Germany or of a biocidal product manufactured here and exported from Germany must submit to the Federal Chemicals Agency for the previous calendar year
- the type and quantity of biocidal products supplied or exported to recipients resident or established in Germany, and
- the active substances contained in the biocidal products supplied or exported.
The notification shall be made separately for each biocidal product and shall include the trade name, the registration number pursuant to the ChemBiozidDV and the case number assigned upon application or the authorisation number pursuant to Article 22 para. 2 (d) of Regulation (EU) No 528/2012.
F. POP
Regulation (EU) 2022/2400 amending Annexes IV and V of Regulation (EU) 2019/1021 on persistent organic pollutants (POP Regulation) has already entered into force on 29.12.2022. The regulation introduces limit values in waste for some substances or tightens already existing limit values. The regulation will apply from 10.06.2023.
Conclusion and outlook
The year 2023 will bring far-reaching legal changes that will present companies with considerable challenges in implementing the regulatory requirements in the area of chemicals law. One particular requirement will be to keep an eye on the various regulatory developments beyond the narrower scope of chemicals law and their impact on production processes, supply chains and marketability requirements. In particular, the expansion of substance-related regulatory efforts, such as in the context of eco-design requirements, will be of great significance for many products with regard to “material compliance”.
Do you have any questions about this news, or would you like to discuss it with the author? Please contact: Martin Ahlhaus






