A. REACH
2022 will be a particularly important year for the application and further development of Regulation (EC) No. 1907/2006 (REACH). In addition to a large number of adjustments and additions to the existing legal framework, the current year will be dominated by the upcoming revision of the regulation.
I. Deadlines
The REACH Regulation itself already provides for a number of specific deadlines that will have to be observed in the current year.
1. Examination of testing proposals
Art. 43(2)(c) REACH, for example, stipulates that by 01.06.2022, corresponding draft decisions will be prepared by the European Chemicals Agency (ECHA) in accordance with Art. 40(3) REACH for all registrations concerning phase-in substances with testing proposals received by 01.06.2018. This means that a number of registrants are likely to face corresponding procedures in the current year.
2. Candidate list for authorization
As early as 17.01.2022, ECHA has added four further entries to the candidate list for the authorization procedure under REACH:
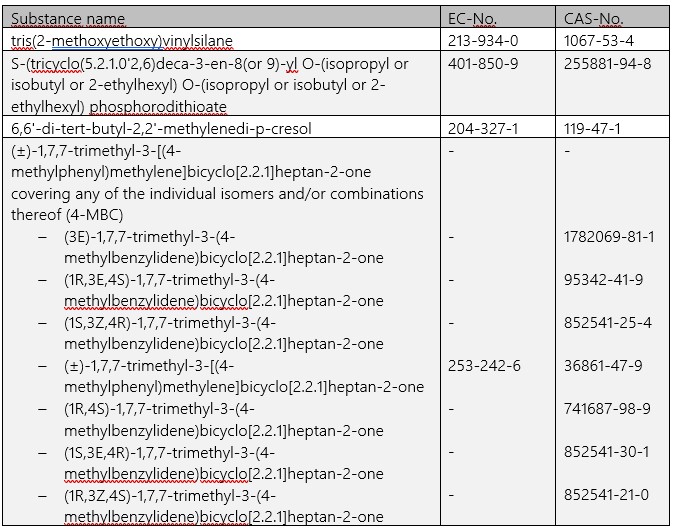
The list now comprises 223 entries, which must be taken into account directly both for communication in the supply chain in accordance with Art. 33 REACH and for notifications in the SCIP database. For any notification obligations under Art. 7(2) REACH, a transition period of a further six months will initially apply to the substances newly included as of 17.01.2022 (cf. Art. 7(7) REACH).
In addition, the entry of PFDA has been amended. The previous group entry “Nonadecafluorodecanoic acid (PFDA) and its sodium and ammonium salts” previously comprised two entries with “Decanoic acid, nonadecafluoro-, sodium salt” and “sodium nonadecafluorodecanoate”. However, as these two entries refer to the same substance, the entry “Decanoic acid, nonadecafluoro-, sodium salt” was deleted.
In line with ECHA’s usual practice, a further addition to the candidate list is expected in the middle of the year.
3. Applications for authorization
For a total of five substances subject to authorization under Annex XIV to REACH, the deadline for submitting applications for authorization ends in the current year. The substances concerned are:
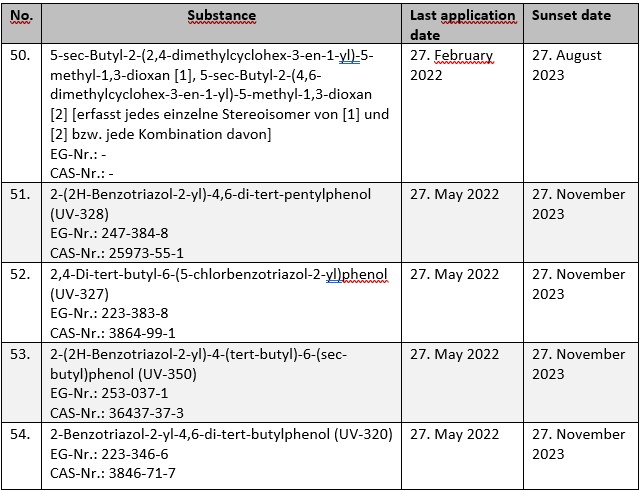
Without a timely application, the substances in question may only be used until the respective sunset dates. This also applies if an application for authorization is submitted after the last application date. In this case, the approval decision must first be awaited before the corresponding use may be resumed.
4. Restrictions
Two of the more recent restrictions are also due to come into force in 2022. For example, according to entry No. 74 in Annex XVII to REACH, diisocyanates may not be placed on the market as a substance or as a constituent in other substances or mixtures for industrial or professional use as of24.02.2022, unless the concentration of diisocyanates individually and in combination is less than 0.1% by weight or the supplier provides more extensive information on safe use and indicates on the label that appropriate training must be provided prior to industrial or professional use as of 24.08.2023.
Use bans for certain substances in tattoo inks have also been in place since 04.01.2022. Details on this can be found in our blog-post dated 05.01.2022.
II. Further amendment of the information requirements
A first package of amendments to the information requirements for registrations in Annexes VII to XI of REACH was already implemented with Regulation (EU) 2021/979. The regulation is already in force since 08.07.2021 and applies since 08.01.2022. The main objective of the readjustment of individual data requirements was primarily to clarify and specify the specifications.
In the meantime, the REACH Regulatory Committee has also unanimously approved the second package of amendments to the information requirements in Annexes VI to X to REACH in the written procedure. In addition to linguistic clarifications, the data requirements relating to CMR properties in particular are to be made more precise. The corresponding draft regulation is available to the Council and the European Parliament for consideration until 19.02.2022. Thereafter, the Commission may publish the draft in the Official Journal of the EU. After entry into force, a transition period of six months is also envisaged here. As with the previous regulation, this amendment to the annexes of the REACH Regulation will not only have to be taken into account when registration dossiers are first prepared and submitted. Rather, the changes will also have to be taken into account when updating existing registrations.
III. Further risk management measures
In the current year, a key aspect of REACH implementation will be the continuation of ECHA’s integrated regulatory strategy. The Assessment of regulatory needs list published by ECHA currently contains 714 entries (as of 13.01.2022). It is also expected for 2022 that ECHA will contact affected registrants of substances for which new risk management measures are expected. Periodically, registrants will be asked to update their registration dossiers and review use information. In addition, registrants will be enabled to anticipate the potential impact of proposed measures.
Currently, for example, a consultation process is still ongoing under the auspices of the German Federal Institute for Occupational Safety and Health (BAuA) to prepare an RMO analysis on fiber materials (substances/mixtures/products) containing particles with a length greater than 5 μm, a diameter less than 3 μm, and a length-to-diameter ratio greater than 3:1 (WHO fiber criterion), and which are biodegradable.
IV. Ongoing consultation procedures
In line with the wide range of potential regulatory approaches, companies and associations should continue to follow the various consultation processes with great care and attention in 2022. This applies in particular to calls for comments and submission of evidence, future restrictions and authorization obligations, as well as any testing proposals. Only with appropriately targeted participation in the corresponding consultation procedures by the industry, regulatory undesirable developments can be avoided.
V. Revision of the REACH Regulation
However, the most far-reaching changes will certainly be associated with the revision of the REACH Regulation. The aim is still for a Commission draft for the implementation of the adjustments to be available by the end of 2022. In the course of the year, there will be a multitude of opportunities and necessities for the participation of industry representatives.
The main objectives of an adaptation of the REACH Regulation follow the “Chemicals Strategy for Sustainability”. Consequently, measures for the targeted implementation of requirements for so-called “safe and sustainable by design chemicals,” for the introduction of “non-toxic material cycles” and the establishment of an “essential use concept” will be taken. In addition, the most hazardous substances as well as endocrine disruptors will be considered, as well as evaluation criteria for mixtures.
As a cross-cutting issue, the “one substance, one assessment” approach will merit special attention for a number of product law requirements. To this end, a horizontal proposal for the reallocation of tasks in the area of the EU agencies concerned, EFSA, ECHA, EMA and EEA, is planned for 2022.
It should also not be overlooked that the chemicals strategy also envisages a “zero tolerance” policy for violations of existing requirements. To this end, enforcement measures are to be strengthened, both at the external borders of the EU and at the level of individual Member States.
B. CLP
Within the framework of Regulation (EC) No. 1272/2008 (CLP), in addition to the ongoing update of harmonized classification and labeling requirements, the focus is primarily on the adaptation and amendment of the regulation itself.
A first key project in this context is the public consultation on the European Commission’s initiative to simplify and digitize the labeling regulations for chemicals. The consultation will run until 16.02.2022.
In addition, the envisaged revision of the CLP Regulation will mainly focus on the introduction of new hazard classes (EDs, PBTs, etc.), the improvement of classification (harmonized classification as well as self-classification), the adaptation of labeling provisions (fold-out labels, exemptions for small packaging, etc.), the requirements for online sales and a possible adaptation of the scope of the CLP Regulation. Due to the far-reaching implications, companies should carefully follow the relevant developments.
C. Biocides
For the current year, the focus will be on continuing procedures concerning the approval of active substances in order to achieve the objectives of the work program. In addition, ongoing procedures concerning the approval of biocidal products at national and European level will of course have to be continued.
I. Product authorisations
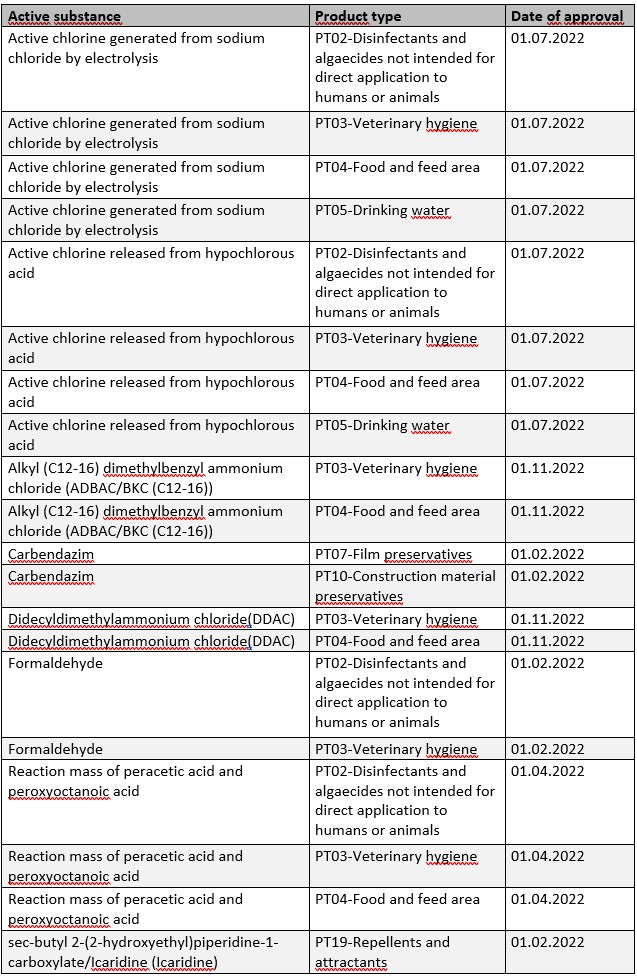
Within the scope of Regulation (EU) No. 528/2012 (BPR), the approval period for a number of active substances will also start in 2022. By the respective date of approval, corresponding applications for authorization of biocidal products must be submitted by affected companies in order to benefit from further transitional periods (cf. Art. 89 BPR).
II. Notification of biocidal products
From 01.01.2022, a new notification procedure will apply to biocidal products in Germany. The previous ChemBiozidMeldeV has been replaced for this purpose by the new Biocide Law Implementation Ordinance (ChemBiozidDV, BGBl. I 2021, No. 57). On this basis, in the future not only biocidal products with existing active substances, which continue to be marketable in Germany without authorization due to an applicable transitional regulation, are subject to notification. Biocidal products made available on the market will also be covered by the reporting obligation in the future. According to BAuA, a correspondingly revised, new online portal will be available from 26.01.2022.
The notifications for biocidal products that fall under the transitional regulations for existing active substances will be taken over from the previous notification register. The new information requirements introduced by the ChemBiozidDV must be fulfilled by 31.03.2022 for such old notifications from the time before 26.08.2021. The new information requirements cover in particular the following:
- the specification of the active substance concentration in the biocidal product
- Information on the application pursuant to Sec. 28 para. 8 2nd Sentence ChemG
- Information on Art. 95 BPR
- Confirmation of the effect attributed to the biocidal product.
D. Conclusion and Forecast
Overall, companies will thus continue to face considerable challenges in 2022 in implementing the regulatory requirements in the area of chemicals law. In the current year, a particular challenge for affected companies and associations will also and especially be to keep an eye on the diverse consultation processes and efforts to make changes as part of the Chemicals Strategy for Sustainability.
Do you have any questions about this news, or would you like to discuss the news with the author? Please contact: Martin Ahlhaus






