This article presents the basic scope of application of the new restriction (see A.), existing exemptions and transition periods (see B. to D.) as well as newly introduced information requirements (see E. and F.). The full text of the regulation is available HERE.
A. Scope of application (column 1 and column 2 para. 1).
Beyond specific regulations and requirements, it follows from the restriction as of the date of its entry into force that synthetic polymer microparticles may no longer be placed on the market as such or, if the synthetic polymer microparticles are present to impart a desired property, in mixtures in a concentration of ≥ 0.01% by weight (cf. paragraph 1 of entry No. 78 in Annex XVII to REACH).
Synthetic polymer microparticles are solid polymers that meet both of the following conditions:
- are contained in particles and constitute at least 1 % by weight of those particles; or build a continuous surface coating on particles;
- at least 1 % by weight of the particles referred to in point (a) fulfil either of the following conditions:
- all dimensions of the particles are equal to or less than 5 mm;
- the length of the particles is equal to or less than 15 mm and their length to diameter ratio is greater than 3.
However, degradable or water-soluble polymers and natural polymers that have not been chemically modified are excluded. Similarly, polymers that do not contain carbon atoms in their chemical structure are not covered by the scope.
The restriction solely prohibits the marketing of synthetic polymer microparticles as such or in mixtures. However, the regulation does not contain a provision that covers such particles in or on articles and would prohibit the placing on the market of these articles. The distinction between substances/mixtures on the one hand and articles on the other hand is to be made according to the known criteria in accordance with the ECHA Guidance on the requirements for substances in articles. For example, textiles or shoes with decorative glitter will regularly be classified exclusively as articles, so that the restriction does not apply to such products.
B. Transitional periods for goods already placed on the market (column 2 para. 16)
Unlike many other restrictions under REACH, the restriction on synthetic polymer microparticles does not provide for a general deferral of the start of application. The restriction thus applies immediately upon its entry into force. However, this does not mean that a sales ban is also immediately associated with it. Paragraph 16 of entry No. 78 in Annex XVII to REACH clarifies – also and especially in interaction with recital (60) to Regulation (EU) 2023/2055 – that products covered by the scope of application of the restriction (also e.g. loose glitter) continue to be marketable insofar as they were placed on the market (for the first time) before 17.10.2023.
This means that, in principle, any goods at all stages of the supply chain can continue to be distributed, i.e. placed on the market, after 17.10.2023 (cf. Art. 3 No. 12 REACH), provided that they were already placed on the market before this date at upstream stages of the supply chain within the scope of the REACH Regulation. It is therefore sufficient, but also necessary, that the goods were transferred by one actor in the supply chain to another before 17.10.2023 or were imported into the scope of the REACH Regulation before 17.10.2023. Under these conditions, stockpiles therefore also continue to be marketable.
However, affected companies should of course be able to document with sufficient precision that and which specific products were already placed on the market before 17.10.2023 and therefore do not lose their marketability after this deadline (e.g. via batch numbers, delivery bills, inventory data as of the deadline or similar).
C. Application-specific and product-specific exemptions
The restriction also contains some general exemptions, such as for uses in industrial plants (with more extensive information requirements from 17.10.2025 and additional reporting requirements), pharmaceuticals, EU fertilizer products, food additives, in vitro diagnostics, or even food and feed (column 2 para. 4).
In addition, the restriction would not apply insofar as the particles are contained by technical means in such a way that release into the environment is prevented if they are used as prescribed during the intended end use, such as in ion exchange resins, diapers or sanitary napkins. In addition, such synthetic polymer microparticles remain exempt from the restriction if their physical properties are permanently modified during their intended end use such that the polymer is no longer within the scope of this entry. Synthetic polymer microparticles that are permanently incorporated into a solid matrix during the intended end use also remain exempt. Particles in mixtures that cure or otherwise solidify during the end use are mainly covered here (column 2 para. 5).
However, since in these cases releases of synthetic polymer microparticles can nevertheless not be completely ruled out, instructions for the use and disposal of some of these products in particular must be made available to professional users and the general public from 17.10.2025 or 17.10.2026 onwards, explaining how the release of synthetic polymer microparticles into the environment can be prevented (column 2 paras. 7 and 8).
The manner of fulfilling this additional information obligation is only described in rather general terms in the restriction. In any case, an indication in the form of clearly visible, legible and indelible text or in the form of pictograms is required. The information can be given on a label, the packaging or in the package insert. If a safety data sheet is required under Art. 31 REACH, the information must be included there. Supplementary (but not exclusive) use of digital access forms also remains possible. Unless pictograms are used, the relevant instructions are to be written in the official languages of the Member States in which the substance or mixture is placed on the market. However, the Member States may also make different arrangements in this respect, for example with regard to the acceptance of other language versions or the requirement for multilingual instructions (column 2 para. 10).
However, it is not required in this context that an explicit reference is made to the fact that the respective product contains microplastics. This requirement exists only for certain cosmetic products (column 2 para. 9).
D. Product-specific and use-specific transition periods
Cosmetic products in particular, along with other products, are covered by a variety of – sometimes long – transition periods, so that the restriction does not apply until a later date and corresponding products containing synthetic polymer microparticles are not subject to the restriction until the transition periods thus created have expired. These transition periods are contained in column 2 para. 6 as follows:
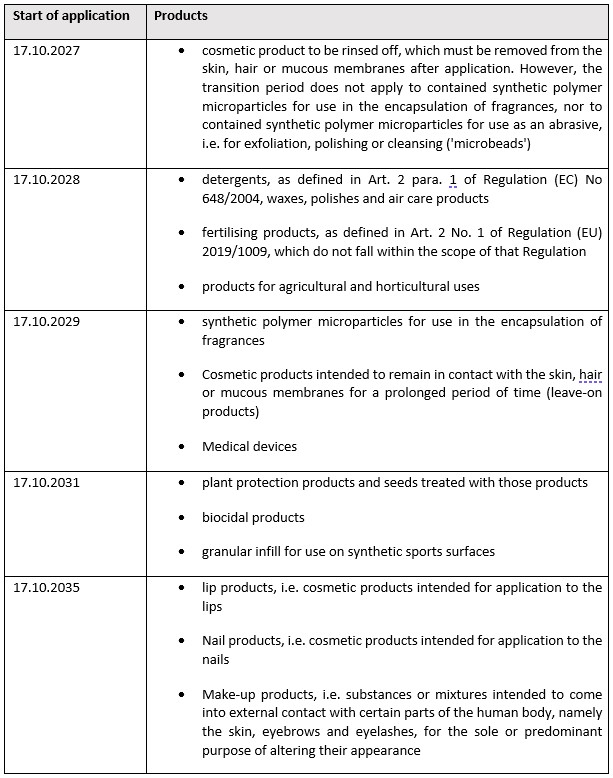
The extended transition periods apply only to the extent that the products in question cannot be assigned to other transition periods ending earlier. Products that could be assigned to different transition periods in this respect will in principle be subject to the restriction on the earlier date in each case.
E. Information requirements vis-à-vis ECHA (column 2 para. 12)
From 2027 onwards, suppliers of medicinal products and veterinary medicinal products, food additives and in vitro diagnostics, as well as products containing synthetic polymer microparticles but exempted from the restriction because they are entrapped by technical means or their physical properties are permanently altered during the intended end use or they are integrated into a solid matrix, must fulfill more extensive information obligations towards the European Chemicals Agency (ECHA).
In addition to describing the end uses for which the synthetic polymer microparticles were placed on the market in the respective previous calendar year, general information on the identity of the polymers placed on the market in the previous calendar year must also be submitted for each end use. This is supplemented by an obligation to provide an estimate of the amount of synthetic polymer microparticles released into the environment during the previous calendar year, including the amount of synthetic polymer microparticles released into the environment during transport.
The relevant information is provided to the Member States by ECHA.
F. Information obligation towards enforcement authorities (column 2 paras. 14 and 15)
Finally, the obligation of manufacturers, importers and downstream industrial users of products containing synthetic polymer microparticles to provide the competent authorities, upon their request, with specific information on the identity and function of the polymers covered by the restriction should not be underestimated. This will not be trivial, in particular, because the specific information on the identity of the polymers must be sufficient to uniquely identify the polymers. In this context, the restriction refers to requirements for determining the substance identity according to Annex VI REACH, although the specifications contained therein were introduced for the purpose of registering substances and polymers as such are precisely not subject to registration (cf. Art. 6 para. 3 REACH). Notwithstanding this, the following information must be made available at the request of the authorities:
- Name and other designations of the substance
- Name(s) according to IUPAC nomenclature; if not available, other international chemical name(s).
- Other names (common name, trade name, abbreviation)
- EC number, i.e. Einecs, ELINCS or NLP number or number assigned by the Agency (if available and relevant)
- CAS designation and CAS number (if available)
- Other identification code, e.g. customs number (if available)
- Information on molecular and structural formula or crystal structure of the substance
- Molecular and structural formula (including Smiles notation and other representation, if available) and description of crystal structure(s)
- Information on optical activity and typical proportion of (stereo)isomers (if applicable and relevant)
- Molecular weight or molecular weight range
- Any qualitative analytical data required for the identification of the substance, e.g. ultraviolet, infrared, NMR, mass spectrography or diffraction data.
- Any quantitative analytical data required for identification of the substance, such as chromatographic, titrimetric, or elemental analysis data, or diffraction data
- Description of analytical methods or indication of bibliographic data necessary for the identification of the substance (including identification and quantification of its constituents and, where appropriate, impurities and additives). The description shall consist of the underlying experimental protocols and the corresponding evaluation of the results referred to in points 2.3.1 to 2.3.6. The information shall enable the methods to be reproduced.
The obligation applies immediately and without any further transitional period. It should be noted, however, that the obligation does not apply to downstream industrial users. Pure distributors are also not affected, as they are not covered by the term “downstream user” according to Art. 3 No. 13 REACH. Specific requests for information in the context of general market surveillance measures in retail or online trade should therefore not be expected.
Moreover, the relevance of the corresponding duty to provide information is reduced even at the level of downstream users. If the information is not available there, they only have to request the information from their supplier within seven days of receipt of the request and inform the authority without delay. In this case, the supplier’s obligation is exhausted by the request to the supplier. The latter must then transmit the requested information within 30 days directly to the competent authority that requested the information or else pass it on to the downstream industrial user, who must then forward it to the competent authorities without delay. Conversely, if the authority receives the information directly, it shall inform the downstream industrial user.
G. Conclusion and outlook
The restriction of synthetic polymer microparticles is characterized by a high degree of complexity due to the regulatory system – general ban with specific, multi-layered exemption and transitional regulations. It is likely that this approach will be adopted for a variety of other restrictions in the future, as vividly highlighted by the PFAS restriction proposal, for example.
For numerous products containing synthetic polymer microparticles, the restriction offers exemptions or longer transitional arrangements. Therefore, despite the basically immediate start of application, products containing “microplastics” will not be immediately removed from the market. However, it is to be expected that the concrete pressure to act, also and especially in connection with supplementary information and reporting obligations, will make even uses that continue to be permitted increasingly unattractive, and in numerous sectors and supply chains the availability of corresponding products will be limited even before the expiry of transitional periods.
Do you have any questions about this news, or would you like to discuss it with the author? Please contact: Martin Ahlhaus






